Describe the Unique Qualities of Carbon
These factors combine into a unique structure that allows it to easily make a chain of carbon atoms. It have the tendency to form wide network with atoms or molecules.

Carbon Structure Of Carbon Allotropes Britannica
With four valence electrons carbon can covalently bond to oxygen hydrogen and nitrogen to form the many molecules important for cellular function.

. Carbon has a valency of four so it is capable of bonding with four other atoms of carbon or atoms of some. Carbon can exist in several allotropes including graphite diamond amorphous carbon fullerines and nanotubes. The structures of eight allotropes are shown at the bottom of this page.
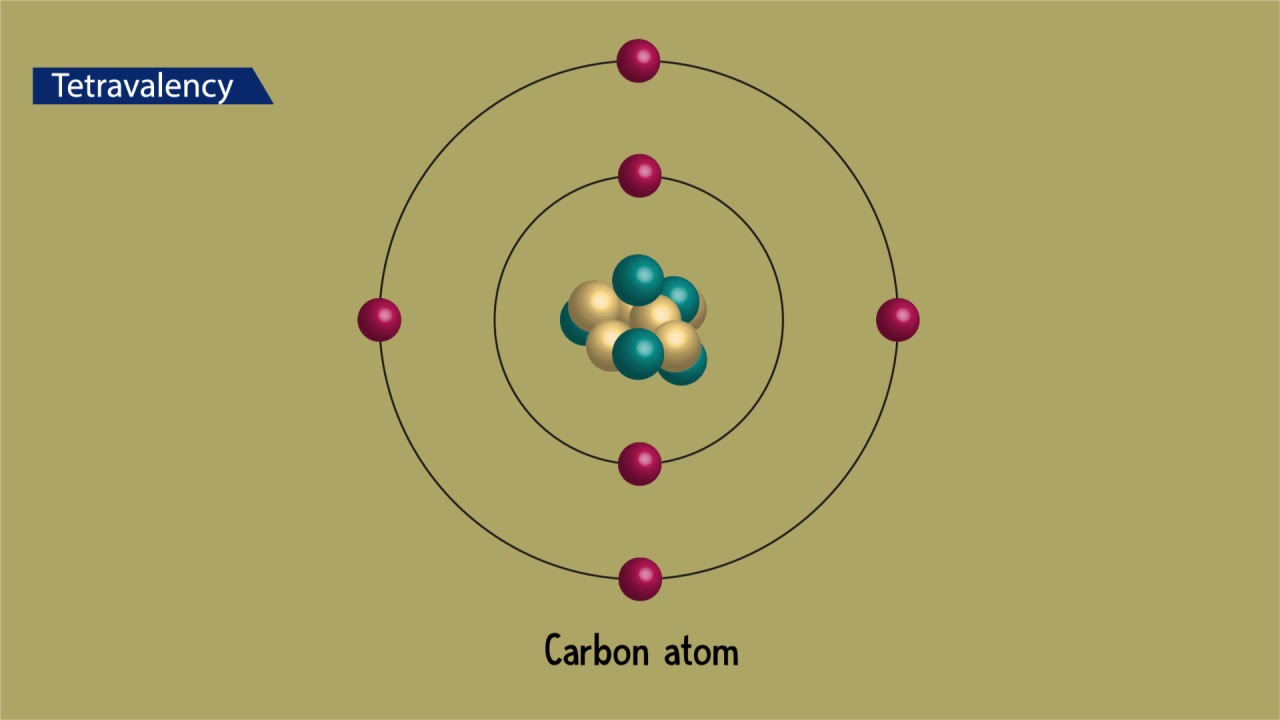
Describe the unique characteristics of carbon that make it the basis of such a large number of organic compounds. The versatile nature of carbon can be best understood with its features such as tetravalency and catenation. One carbon atom can bond to another to form chains and rings.
This type of bond is stronger than an ionic bond where electrons are donated to. The small size of the carbon atom makes the compounds of Carbon exceptionally stable. What makes carbon unique is its ability in forming covalent bonds which are very strong in nature.
Single Walled Carbon Nanotubes Structure. These hydrocarbons extracted naturally as fossil fuels coal oil and natural gas are mostly used as fuels. Carbon have the property of catonation means forming long chain with other atoms or atom by itself it have tendency to form four covalent bond by sharing the outermost electrons.
One carbon atom can bond to another to form chains and rings. We know enough now to be sure that the idea of a world in which silicon should take the place of carbon as the basis of life is impossible. Carbon is unique among the elements in the number and variety of the compounds which it can form it is the basis of all forms of living matter.
The ability of carbon atoms to form covalent bonds with other carbon atoms is the most unique of its bonding properties. Practice Significance of Carbon. Carbon can form millions of different large.
It has four valence electrons and is a very small atom so it always forms four strong covalent bonds. The special nature of carbon combines with the molecular perfection of single-wall CNTs to endow them with exceptional material properties such as very high electrical and thermal conductivity strength stiffness and toughness. Carbon is an element that has unique properties exceptional ability to form bonds that makes it essential to life on earth.
It has four valence electrons and is a very small atom so it always forms four strong covalent bonds. Living organisms are made up of molecules made of carbon and these other elements. The answer lies with carbons unique properties.
It is a versatile element and is found in many different chemical compounds. Carbon is a unique element. No other element in the periodic table bonds to itself in an extended.
Carbon is small and has four valence electrons. One of the most important compounds of carbon is. This indicates how strong.
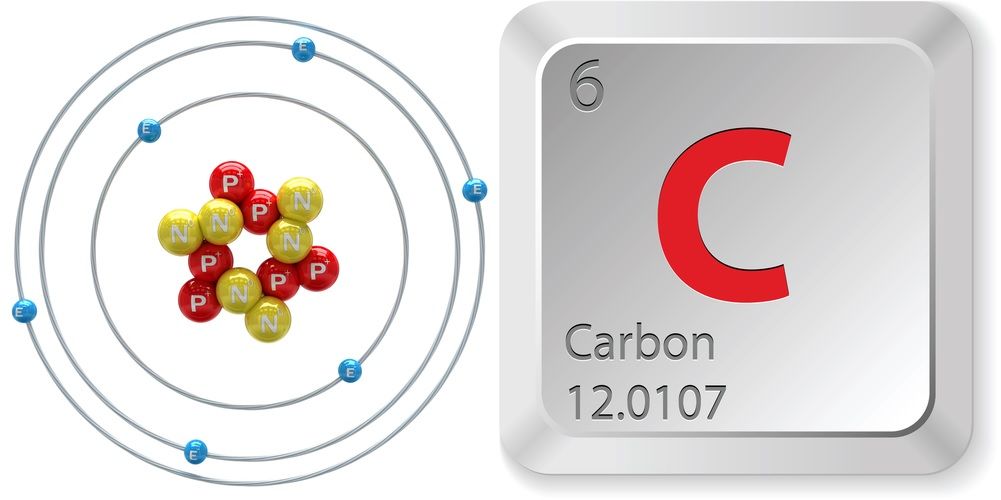
Carbon atoms have four valence electrons allowing them to form strong covalent bonds with many other elements including hydrogen oxygen phosphorus sulfur and nitrogen. Carbon is the chemical element with the symbol C and atomic number 6 contains 6 protons in its nucleus. Carbon atoms have four valence electrons allowing them to form strong covalent bonds with many other elements including hydrogen oxygen phosphorus sulfur and nitrogen.
Carbon is a key chemical element for life and the natural processes that take place on earth. Carbon makes four electrons available to form covalent chemical bonds allowing carbon atoms to form multiple stable bonds with other small atoms including hydrogen oxygen and nitrogen. Carbon is a chemical element with symbol C and atomic number 6.
Moreover it is the only element which could occupy such a position. Its ability to form polymers makes it an ideal partner for molecules that generate life. The Uniqueness of Carbon Because each carbon is identical they all have four valence electrons so they can easily bond with other carbon atoms to form long chains or rings.
This enables carbon to form long continuous chains branches and loops consisting of carbon and hydrogen in hydrocarbons and only carbon in carbon allotropes such as C 60. Living organisms are made up of molecules made of carbon and these other elements. Carbon can form millions of different large.
Hence carbon as an element has the ability to form a variety. A covalent bond is one where atoms share electrons to form a bond. The most common isotope of carbon has 6 protons and 6 neutrons and has an atomic mass of 120107 amu.
The ability of carbon to bond with itself is called catenation. It is soft and dull grey or black in colour. Some examples of the pure form of carbon are coal and soot.
The Importance of carbon in living things Is based on the fact that it is the chemical element on which the existence of life is based. Carbon is unique among the elements in its ability to form strongly bonded chains sealed off by hydrogen atoms. It occurs in many forms.
Inhalation of large quantities of carbon black dust sootcoal dust can cause irritation and damage to the lungs. Carbon has an exceptional ability to bind with a wide variety of other elements. As a member of group 14 on the periodic table it is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bonds.
In fact a carbon atom can bond with another carbon atom two or three times to make double and triple covalent bonds between two carbon atoms. 231 Describe the unique qualities of carbon Carbon atoms have 4 valence electrons allowing them to form strong covalent bonds with many other elements such as hydrogen oxygen phosphorus sulfur and nitrogen to form the molecules of life. The unique properties of carbon make it a central part of biological molecules.
Physical Properties of Carbon. Describe the unique characteristics of carbon that make it the basis of such a large number of organic compounds. It creates covalent bonds the strongest bonds between atoms.

Carbon Compounds Chapter 2 Section 3 Part 1 Objectives Describe The Unique Qualities Of Carbon Describe The Structures And Functions Of Each Of The Ppt Download
What Characteristic Of Carbon Makes It Essential To Living Organisms Lisbdnet Com
Carbon Facts About An Element That Is A Key Ingredient For Life On Earth Live Science
No comments for "Describe the Unique Qualities of Carbon"
Post a Comment